Catalysis and Membranes
There is extensive experience with diverse catalytic reactions as steam reforming of methane, partial oxidation of methane to synthesis gas, aromatization and dimerization of methane, synthesis of dimethyl ether and dimethyl carbonate, oxidative and non-oxidative dehydration as well as Andrussow and Ostwald reactions.
Experience in the field of gas separation membranes is particularly with membranes based on zeolithes, metal-organic frameworks (MOFs) and covalent-organic frameworks (COFs). Moreover, scale-up friendly mixed matrix membranes with MOF or COF nanoparticles as fillers in a pure polymer matrix are investigated as gas separation membranes.
Achievements are published in 360 scientific papers, 42 patents and have been recognized by national (Ostwal Medal) and international (Breck Award) awards. Research has been funded in collaborative projects by EU, BMBF, BMWi, DFG and directly by companies. Partly, investigations have been made at the European Nanoporous Materials Institute of Excellence (ENMIX).
Switching gas transport in a membrane by external stimuli
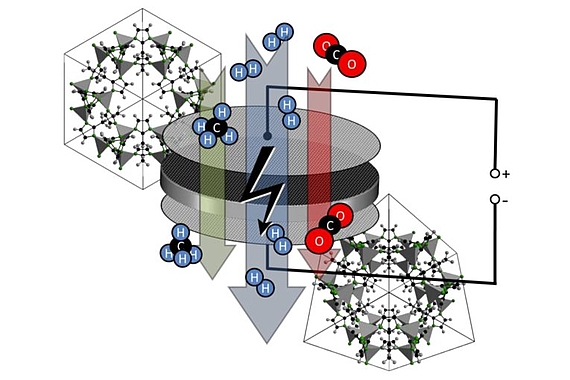


Two variants of switching the gas flux through a MOF membrane have been successfully realized:
An external electrical field acts on ions and dipols in the MOF structure, and it can be used to deform the crystal lattice and thus to control the gas flux through a MOF-based membrane.
Shining light of adjustable wavelength on the membrane surface, azo groups in the MOF membrane can be switched from trans to cis an vice versa. Thus, the pore size and so the gas flux can be varied.
Aromatisation of methane in membrane reactors
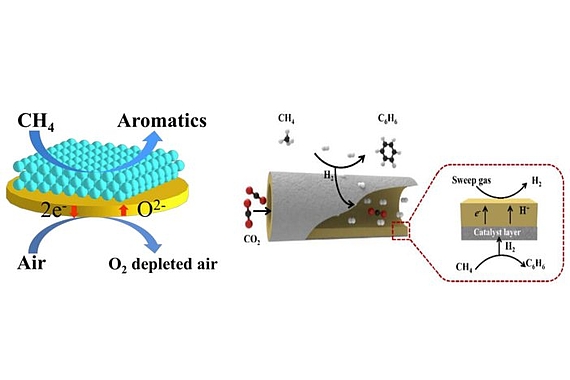


The conversion of methane into liquid energy media and chemicals is highly relevant. One of the routes pursued is methane aromatisation after 6 CH4 → C6H6 + 9 H2 at T>800°C. However, the yields achieved so far are far below 10%. Membrane reactors, however, can solve this problem by allowing them, under equilibrium-controlled conditions, to separate the split-off hydrogen
- removing the separated hydrogen from the reaction chamber through a newly developed hydrogen-selective ceramic membrane
- burning in situ by a stoichiometric amount of oxygen and feeding this oxygen to the reaction chamber through a newly developed oxygen-selective membrane made of air.
Both variants have been successfully practised within the framework of national and international collaborative research under the leadership of LUH by universities, non-university research institutions and industry.
Contact


30167 Hannover




