Development of model systems for the computer-aided investigation of phase boundaries, composite materials and molecules
The group investigates reactions and structure formation at phase boundaries and the dynamics of these processes. Our approach is to simplify the complex issues in model systems in order to be able to analyze the various factors influencing the processes separately at the molecular level. We use ab initio simulation programs to obtain detailed information about the quantum chemical and thermodynamic properties of the molecules and structures. On the other hand, the interaction of different components at a phase boundary also requires a description over a certain temporal and spatial size scale, which is why we also use atomistic force field models based on quantum chemical programs.
In order to validate the theoretical findings, specially developed experimental apparatus and methods adapted to the demanding reactive conditions are used. The aim is to gain a better understanding of the dynamics, reactions and transport phenomena in molecules, nanoparticles and interfaces.
Theoretical investigations of complex interfaces and their influence on crystal growth
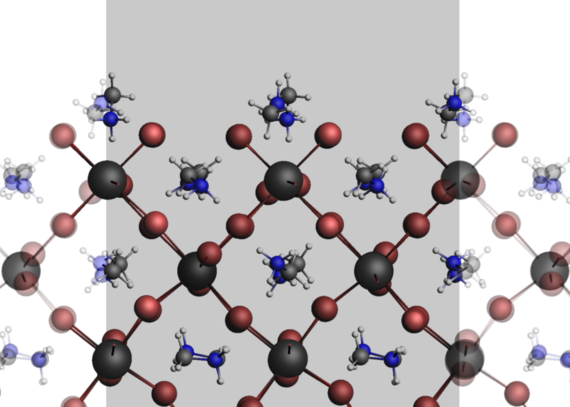

In collaboration with industry and other groups in the Faculty of Natural Sciences, we simulate the interfaces of various components of composite materials, e.g. polymer systems at SiO2 and carbon interfaces, the stabilization of ZnO nanoparticles by ligands or the surface tensions of various perovskites. In particular, ab initio methods (Gaussian and ADF) are used for this purpose. Thermodynamic properties that provide information on the mechanical stability of the elastic materials can be determined from the computational chemistry calculations.
The surface tension during the crystal growth phase has a strong influence on the crystal morphology of the particles. It can be influenced by the use of different ligands or the saturation of the precursors, for example in the synthesis of zinc oxide nanoparticles or the synthesis of MAPbBr3 particles from an aerosol. Findings from simulations on the interactions between the ligands and the nanoparticles as well as the surface tension of various crystal facets support experimental work by other groups, allowing conclusions to be drawn about crystal growth.
Theoretical and practical investigations into the electrochemical synthesis of metallic nanoparticles in organic media
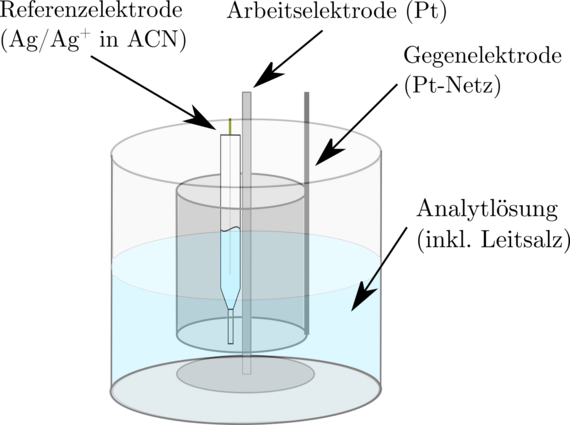
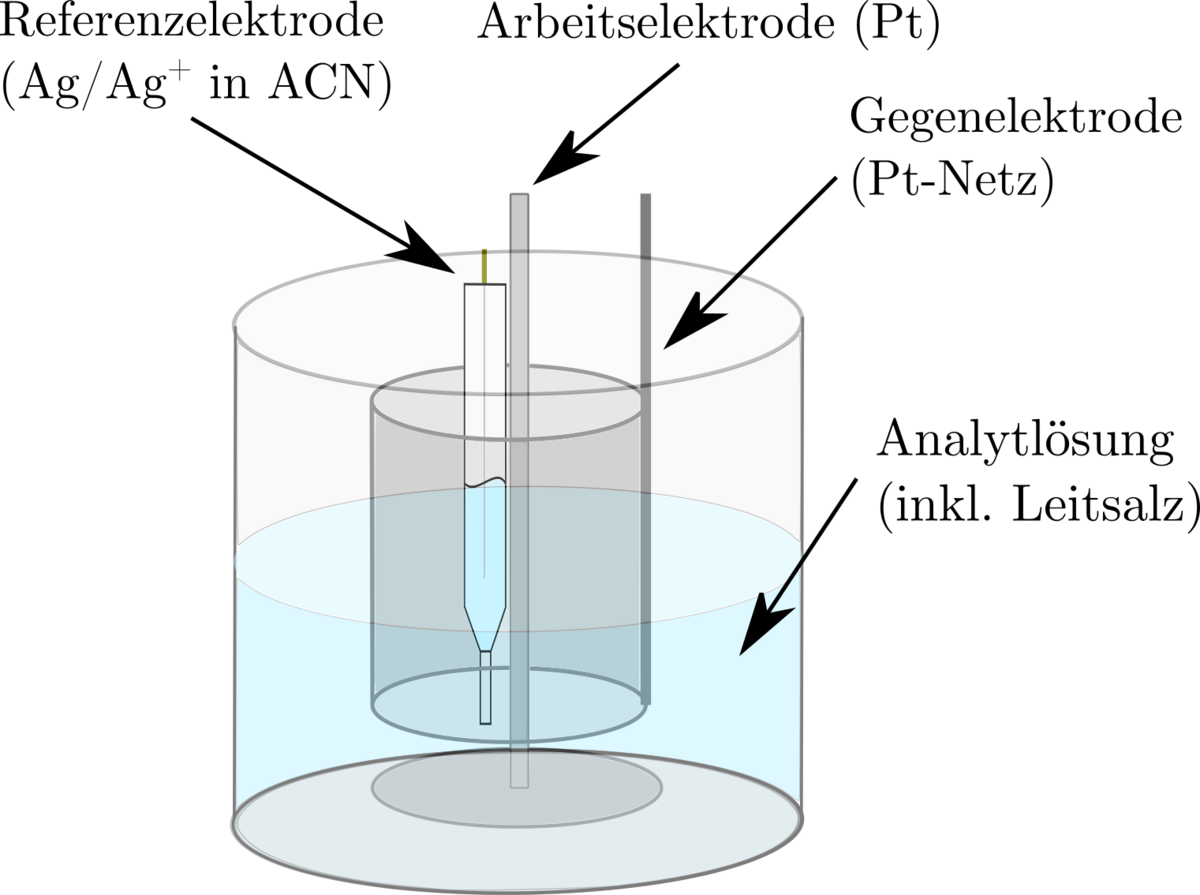
Electrochemical properties are accessible both experimentally and via ab-initio methods. For example, redox potentials can be determined in the laboratory using cyclovoltametry and then compared with the results of quantum chemical simulations. The solvation effects, which significantly influence the molecular properties, are of particular interest here.
These effects are of particular importance at phase boundaries, such as on the surface of nanoparticles or electrode surfaces. The role of solvents, linkers or stabilizers can be mapped using a combination of statistical thermodynamic approaches and ab initio dynamics.
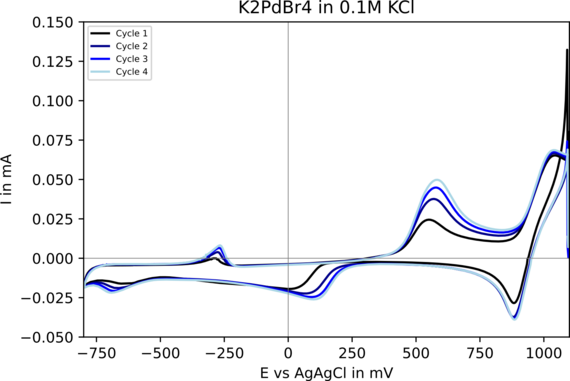
In particular, the electrochemical synthesis of transition metal nanoparticles in organic media is being investigated experimentally. The organic conducting salts used are sterically demanding and prevent the transition metal species formed in situ on the sacrificial anode from being deposited on the working electrode. The species responsible for mass transport are characterised using UV-Vis spectroscopy, IR and Raman spectroscopy and cyclic voltammetry. The finally formed stable colloids are analyzed by vibrational spectroscopy and electron spectroscopy. Further electrochemical analyzes using cyclovoltametry, conductivity, impedance and rotating disc electrodes, together with ab initio investigations, extend the understanding of the processes taking place.



Magnetic properties are also analysed in situ using magnetic metals such as cobalt. Corresponding investigations are carried out in an inert gas atmosphere.
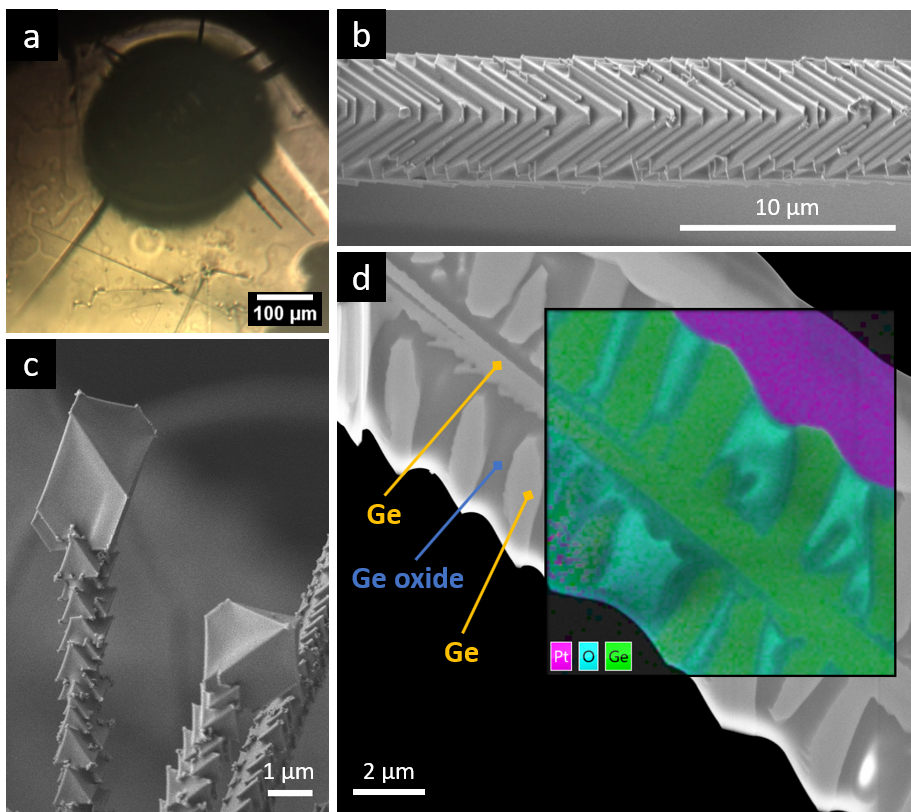
Experimental observation of reactive wetting effects of complex germanium oxide and silicon oxide interfaces
We investigate high-temperature reactions in a high vacuum, the transport of gas reaction products into the environment and their subsequent involvement in the formation of solid structures with new morphology and properties. One example of such structures are complex germanium/germanium oxide microneedles. One of the main features of our research method is that the entire reaction-transport-structure formation chain takes place in an isolated microreactor with a volume of only a few cubic millimeters. The great advantage of such a small reaction space is that the entire available volume can be controlled and observed throughout the process and that a minimal amount of product (in the sub-milligram range) is used, which in turn significantly increases the purity of the experiment.
The demanding, reactive conditions are experimentally challenging. New measuring equipment is therefore being developed and built to adapt in situ analytical methods such as high-temperature video microscopy in the visible and near-infrared spectral range to these conditions.
Ex situ, we use electron microscopy and atomic force microscopy to visualize and measure the topography of the samples produced. In collaboration with other groups from the Faculty of Natural Sciences and Mechanical Engineering, further investigation methods are available, e.g. to analyze the chemical composition of the samples.
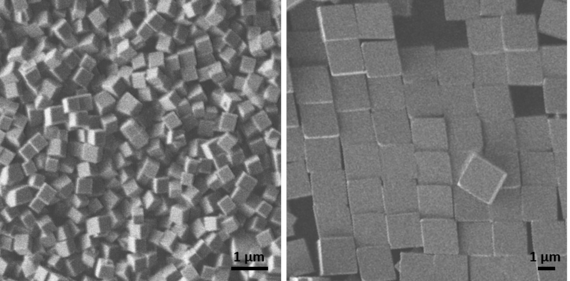
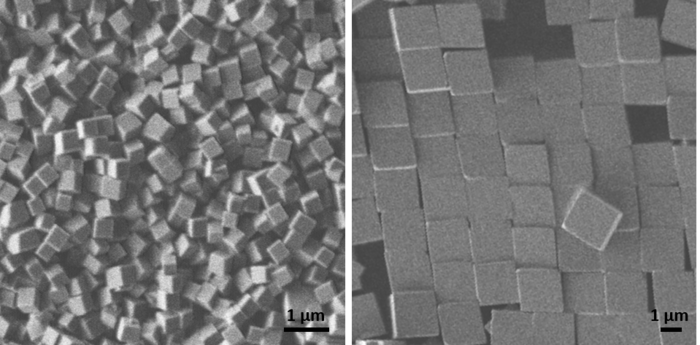
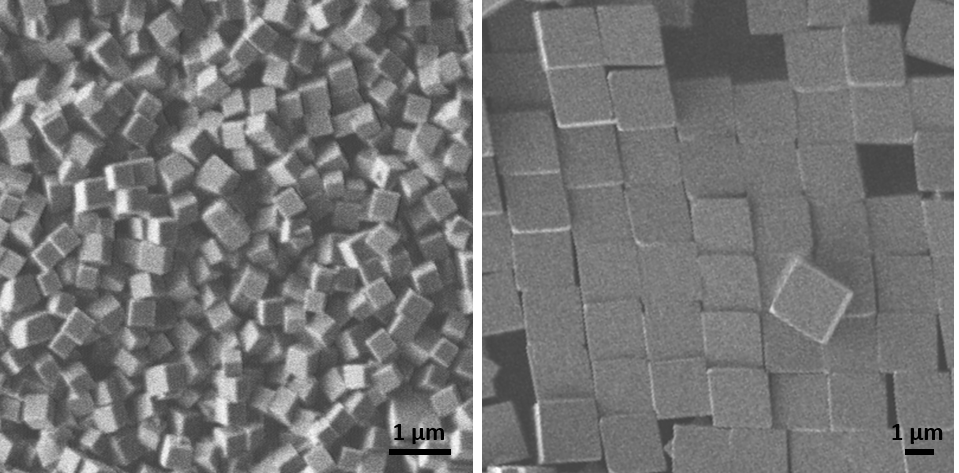
We also grow microscopic GeO2 cubes at room temperature in droplets with a volume of only a few tens of microlitres. This allows the entire volume in which the reaction takes place, and thus the growth of the crystals, to be observed in situ using video microscopy, for which phase-contrast and fluorescence microscopes are also used. The reaction in the droplet can also be analysed directly using an infrared spectrometer. Ex situ we use light and electron microscopy. Thanks to co-operation with other working groups, EDXS and Raman spectroscopy are available, for example.
contact
30167 Hannover